 In 2023, new developments and shifts in the health policy landscape brought a renewed imperative to root decisions in evidence. These included the implementation of the Inflation Reduction Act’s drug pricing provisions, the continued expansion of the 340B program, growing scrutiny of PBM practices, deepening concerns over value assessment practices, and other issues. Learn how NPC took on these complex challenges and helped keep patients at the center of health policy discussions by driving rigorous research and timely analysis, and communicating it to stakeholders.
In 2023, new developments and shifts in the health policy landscape brought a renewed imperative to root decisions in evidence. These included the implementation of the Inflation Reduction Act’s drug pricing provisions, the continued expansion of the 340B program, growing scrutiny of PBM practices, deepening concerns over value assessment practices, and other issues. Learn how NPC took on these complex challenges and helped keep patients at the center of health policy discussions by driving rigorous research and timely analysis, and communicating it to stakeholders.
Keep up with NPC and the latest health policy research, news, and events by subscribing and follow NPC on LinkedIn.
| Subscribe for Updates | Connect With NPC on LinkedIn |
Table of Contents
PRESIDENT'S MESSAGE
 The work of the National Pharmaceutical Council has never been more relevant — or more important. NPC conducts policy-relevant research about the value of biopharmaceutical innovation and communicates it with impact. We provide evidence to bolster the case for the value of innovation in medicine.
The work of the National Pharmaceutical Council has never been more relevant — or more important. NPC conducts policy-relevant research about the value of biopharmaceutical innovation and communicates it with impact. We provide evidence to bolster the case for the value of innovation in medicine.
In 2024, we celebrate our 70th year, and I’m pleased that today we are better positioned to educate leaders about what data and research say than ever before. Our policy-relevant research agenda and communications-forward approach mean we are playing an important role leading conversations in a dynamic health policy environment. Our work has been energized by a leadership team with deep experience in the current battles happening in both commercial and government health policy.
This work is only growing more important. We are living in an age of remarkable innovation and an environment threatened by misinformation that is shaping policy and public opinion. 2024 promises to bring new challenges. Our team has built new capabilities to respond and is actively engaging. We look forward to continuing to increase our impact in 2024 and will continue our 70-year legacy of conducting rigorous and trusted research that stakeholders rely on and value.

John M. O’Brien, PharmD, MPH
President and CEO
NPC AT 70
From our earliest work on patient access to our most recent focus on the Inflation Reduction Act’s Drug Price Negotiation Program, NPC has spent the last 70 years as a leader in health policy research. Over those seven decades, the organization has continued to thrive as we conduct policy-relevant research and communicate it with impact. 
NPC has been ahead of the curve on health policy issues right from the start, with an early focus on drug safety and generic substitution issues 30 years before the Hatch-Waxman Act and long before any kind of labeling or equivalence standards went into effect. Our history contains so many big moments in health policy. The creation of Medicare in the ‘60s. The Hatch-Waxman Act in the ‘80s. The Medicare Drug Rebate Program and the rest of OBRA ‘90. The Medicare Modernization Act in 2003 and the Affordable Care Act in 2010. And most recently, the Inflation Reduction Act in 2022.
We haven’t just witnessed 70 years of change within our industry; we’ve published policy-relevant research that has helped shape the impact of those changes to benefit patients and protect both access to innovation and the ecosystem that supports future innovation.
Over the years, NPC has shaped the pharmaceutical industry in many ways. For decades, we published the Compilation, which tracked pharmaceutical benefits under Medicaid. Archive - Pharmaceutical Benefits Under State Medical Assistance Programs
 We worked to help develop the next generation of leaders by starting an internship program with the American Pharmaceutical Association (a program that launched the career trajectories for numerous professionals, some of whom now sit on NPC committees as representatives of our member companies), and we’ve continued this legacy of work through collaborative fellowships such as the Rutgers-NPC Health Policy & Communications Fellowship.
We worked to help develop the next generation of leaders by starting an internship program with the American Pharmaceutical Association (a program that launched the career trajectories for numerous professionals, some of whom now sit on NPC committees as representatives of our member companies), and we’ve continued this legacy of work through collaborative fellowships such as the Rutgers-NPC Health Policy & Communications Fellowship.
We’ve raised awareness about medication adherence, examined the impact of closed versus open formularies, called attention to the importance of recognizing individual patient differences, published guiding practices for patient-centered value assessment, examined the unintended consequences of policy changes, and raised concerns about barriers to patient access through rigorous and often peer-reviewed research and analysis.
Over the past decade, NPC has worked extensively to help transform the value assessment field and educate stakeholders on the science and judgment that make up health technology assessments. NPC has raised the bar on real-world evidence, recognizing the value of biopharmaceuticals, highlighting the need for increased access to care, and emphasizing the importance of continued innovation across all sectors of our healthcare system.
 We live in a golden age of medical innovation, with biopharmaceutical scientists discovering and developing incredible new treatments that now include durable therapies and even cures. However, the healthcare marketplace is trying to pay for these advances with an outdated payment system, and attempts at policy interventions too often seem guided by political expediency rather than arriving at evidence-driven solutions. Patients need access to these innovations, and we need as much innovation in how the healthcare marketplace pays for medicines as we see in the labs of our member companies.
We live in a golden age of medical innovation, with biopharmaceutical scientists discovering and developing incredible new treatments that now include durable therapies and even cures. However, the healthcare marketplace is trying to pay for these advances with an outdated payment system, and attempts at policy interventions too often seem guided by political expediency rather than arriving at evidence-driven solutions. Patients need access to these innovations, and we need as much innovation in how the healthcare marketplace pays for medicines as we see in the labs of our member companies.
Good policy requires good research questions and methods. NPC will continue to conduct rigorous research and work to educate stakeholders, as it has for the last 70 years, to inform the work to improve patients’ lives and benefit society.
NPC IN 2023

Leading the health policy environment in 2023 was the implementation of the Inflation Reduction Act’s Medicare Drug Price Negotiation Program — but it was far from the only issue. Ongoing debate about pharmacy benefit managers, march-in rights, benefit design, healthcare costs, and drug prices also shaped the conversations — and NPC’s research pipeline.
Amidst such a challenging policy environment, NPC added new leadership to its team — bringing new energy and ensuring the organization was fit for purpose heading into 2024. In July, NPC welcomed Jon Campbell, PhD, as Chief Science Officer and Michael Pratt as Chief Communications Officer. In December, Kimberly Westrich, MA, joined the Executive Leadership team as Chief Strategy Officer. The NPC team drove the organization to increased awareness, engagement, and impact in the second half of 2023 with great momentum heading into 2024.
On the research side, NPC continued focus on three strategic priority areas: to examine and explain the true cost of medicines and the value they provide, address critical issues of value assessment and other approaches used to impact drug pricing and the downstream effect on innovation, and educate policy stakeholders and the public on the underlying issues that impact patient access. NPC closed the year with 9 peer-reviewed publications published or accepted for publication.
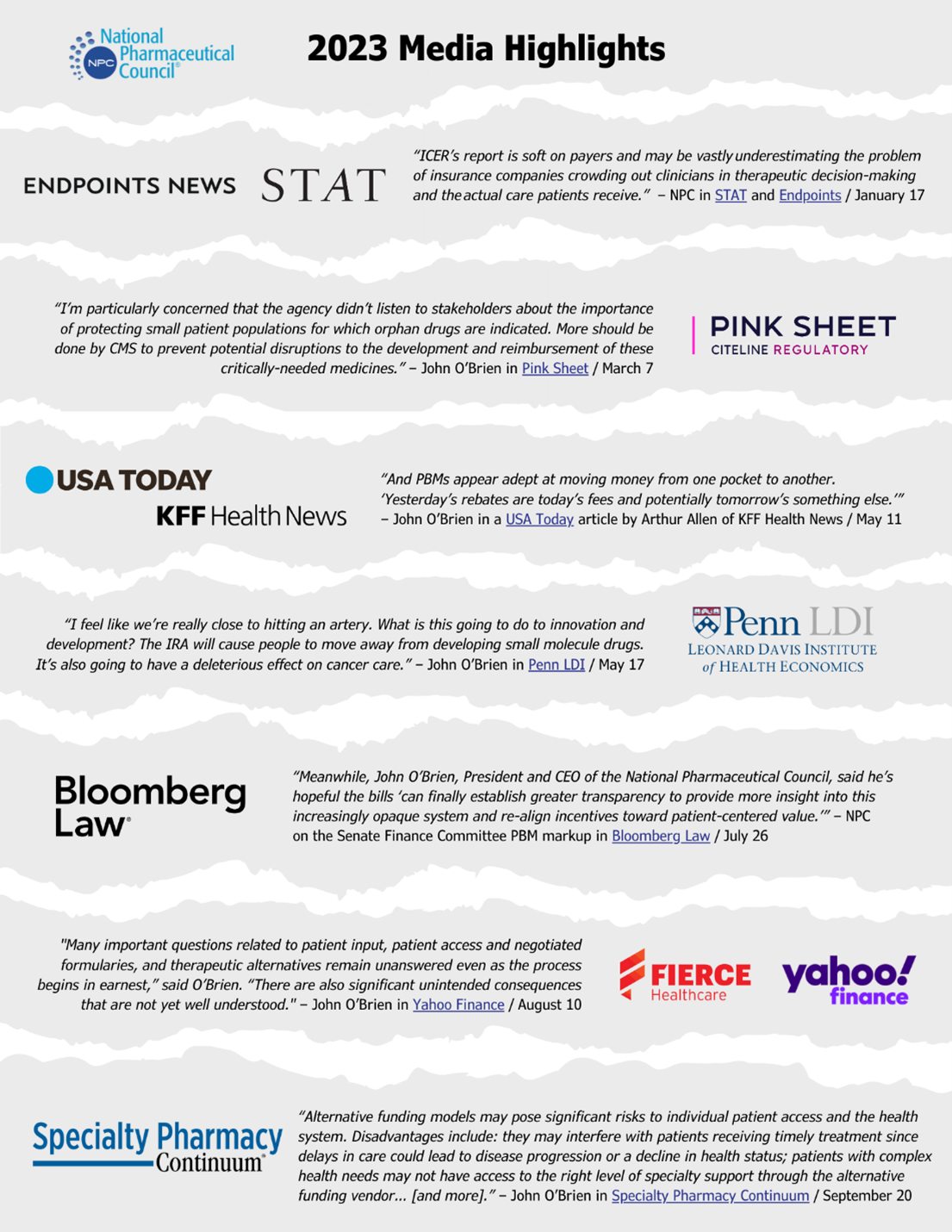
NPC also worked to communicate its research with impact. NPC leaders spoke at key industry events and conferences, published thought leadership and commentary, and worked to share insights with external stakeholders — all leading to a growing number of patient groups, associations, and opinion leaders engaging with our work and sharing it with their audiences. NPC also closed the year with nearly double the number of media mentions in 2023 compared to 2022, including important news coverage related to the IRA.
In December, NPC launched a weekly newsletter, NPC This Week, to provide decision-makers with timely NPC research and analysis related to the policy and industry conversations that are impacting the future of innovation and access.
Finally, NPC returned to its convening roots in a meaningful way in 2023, bringing multistakeholder voices to engage with our research, our team, our Board, and our members.

• Policy-Relevant Research
Highlights of NPC’s research publications in 2023 include:
- A study published in JAMA Health Forum in July examined the role of the National Institutes of Health and industry in bringing new treatments into clinical settings. The paper, “Spending on Phased Clinical Development of Approved Drugs by the US National Institutes of Health Compared With Industry,” was widely cited in the popular press and generated an Altmetric score of 84 (as of December 2023).
- NPC also supported research related to the march-in rights debate, including the highly-cited “March-in rights under the Bayh-Dole Act & NIH contributions to pharmaceutical patents” report in November.
- A study published in Health Affairs Scholar in August examined how health plan policies have become more restrictive over time. “Commercial Coverage of Specialty Drugs 2017-2021” found that commercial health plans’ use of prescriber requirements doubled and use of step therapy increased by more than 31 percent for specialty drugs.
The second half of the year saw an increased focus on the Inflation Reduction Act and its Medicare Drug Price Negotiation Program.
- An analysis published in Health Affairs Forefront examined new drug launches and research after a drug’s initial FDA approval. It presented case studies of three drugs against the backdrop of the timeline for a small molecule drug’s eligibility to be selected for the IRA’s DPNP. “How the Inflation Reduction Act Could Delay Pharmaceutical Launches, Reduce Indications, and Chill Evidence Generation” illustrates how the IRA’s DPNP may impact economic incentives surrounding clinical development in the U.S. and create incentives that delay manufacturers’ decisions to seek initial approvals, discourage research toward subsequent indications, and impact overall survival data.

- In the December 2023 issue of Value in Health, NPC President and CEO John O’Brien joined Genentech’s Jan Hansen in offering considerations for CMS on the implementation of the IRA’s DPNP. In “Section 50 of the Inflation Reduction Act Drug Price Negotiation Program: Considerations for the Centers for Medicare Medicaid Services, Manufacturers, and the Health Economics and Outcomes Research Community,” they outline concerns over CMS’s ambiguous standards and intent to incorporate nonclinical factors unrelated to product value (such as manufacturing costs). The authors warn that these approaches threaten to artificially suppress negotiated prices below fair value and necessitate increased procedural clarity and rigor.
- NPC Chief Science Officer Jon Campbell joined Melanie D. Whittington, Peter J. Neumann, and Joshua T. Cohen for a Health Affairs Forefront piece arguing that practitioners of cost-effectiveness analyses should incorporate dynamic drug pricing into formal cost-effectiveness analyses (CEAs). “The Case for Including Dynamic Drug Pricing in Cost-Effectiveness Analyses Under the IRA” discusses why doing so will more accurately reflect drug costs over time and how the provisions of the Inflation Reduction Act make the inclusion of dynamic drug pricing in CEAs even more important.
• Communicating With Impact
The intense health policy debate over how to make innovative treatments available and accessible to patients creates a noisy environment, often driven by talking points rather than evidence. To help inform a more substantive discussion that can yield sustainable, patient-centered solutions, NPC works to amplify published research and analysis. Our communications and engagement efforts help connect NPC's researchers and experts with journalists covering complex health policy topics, advocacy organizations working to navigate the policy landscape on behalf of the patients they serve, and professionals attending meetings and conferences throughout the year.
While regulatory or professional guidelines can often seem arcane, the success or failure of health policies in improving the care patients can access depends on guidance that reflects the best available evidence and navigates the challenges of real-world implementation. To inform the efforts of regulatory agencies and other organizations, NPC developed and submitted six comment letters responding to comment periods in 2023, distilling important takeaways from NPC’s body of research. Letters were submitted to Health and Human Services (HHS) and the Centers for Medicare and Medicaid Services (CMS), largely focusing on the implementation of the IRA’s Medicare Drug Price Negotiation Program (DPNP) Letters were also submitted to the Institute for Clinical and Economic Review (ICER) on their value assessment framework.
Read NPC's Comments on the CMS Medicare Drug Price Negotiation Program
 In 2023, NPC launched NPC This Week, a timely look-ahead newsletter on the policy, research, and industry conversations that impact the future of biopharmaceutical innovation. We continued to offer the CER Daily Newsfeed®, a daily summary of health policy research activities and news from around the world, as well as new analysis and perspectives through the NPC blog.
In 2023, NPC launched NPC This Week, a timely look-ahead newsletter on the policy, research, and industry conversations that impact the future of biopharmaceutical innovation. We continued to offer the CER Daily Newsfeed®, a daily summary of health policy research activities and news from around the world, as well as new analysis and perspectives through the NPC blog.
NPC’s research and analysis created many opportunities for thought leadership. In 2023, we saw nearly double the number of mentions of NPC in the media and online. Our research and analysis was featured in news coverage of important events such as IRA DPNP Implementation milestones.
We expanded our LinkedIn audience while continuing to reach top stakeholders in government, industry, academia, and the broader healthcare community. These online interactions translated into follow-up engagement and education opportunities with industry experts, researchers, and policymakers looking to leverage NPC’s body of work in pressing policy conversations.
NPC’s leaders, including Drs. O’Brien and Campbell, were also featured speakers at many industry conferences, specialty societies, and academic institutions. Alongside other members of the NPC research team, they highlighted takeaways from NPC’s portfolio of work on a range of topics including pricing, patient access and pharmacoequity, PBM reform, the 340B program, value assessment frameworks, and alternative payment models. This included direct interactions with federal policymakers: At ISPOR 2023, Dr. O’Brien shared the stage with CMS’s Center for Medicare Director Meena Seshamani as part of a panel on “Affordability and Inward Investment – What does it Mean for HEOR?” And at the Annual Forum of the National Alliance of Healthcare Purchaser Coalitions, Dr. O’Brien shared the stage with Commissioner Alvaro Bedoya of the Federal Trade Commission.
 NPC’s engagement with organizations often focused on helping to cultivate the next generation of health policy professionals. NPC has sponsored events and been a frequent guest of student sections of professional organizations, including ISPOR—The Professional Society for Health Economics and Outcomes Research, the Academy of Managed Care Pharmacy (AMCP), the Industry Pharmacists Organization (IPho), and AcademyHealth. Drs. O’Brien and Campbell also served as guest lecturers at several universities, including the University of Pennsylvania, Harvard University, Rutgers University, Tufts University, the University of Colorado, the University of Minnesota, and the University of Miami. Our team has also been actively sharing our research and connecting with future leaders on LinkedIn.
NPC’s engagement with organizations often focused on helping to cultivate the next generation of health policy professionals. NPC has sponsored events and been a frequent guest of student sections of professional organizations, including ISPOR—The Professional Society for Health Economics and Outcomes Research, the Academy of Managed Care Pharmacy (AMCP), the Industry Pharmacists Organization (IPho), and AcademyHealth. Drs. O’Brien and Campbell also served as guest lecturers at several universities, including the University of Pennsylvania, Harvard University, Rutgers University, Tufts University, the University of Colorado, the University of Minnesota, and the University of Miami. Our team has also been actively sharing our research and connecting with future leaders on LinkedIn.
This year, we also saw Hill staff, former government officials, trade associations, prominent industry thought leaders, and leaders from patient advocacy groups and provider organizations circulate, share, and engage with our work, encouraging others to read and use our research and comment letters. NPC believes its research must be used in order to be useful, and will continue to build capabilities and programming to educate more decisionmakers about its research in 2024.
MEMBERSHIP AND BOARD
NPC is a member-based nonprofit organization supported by leading biopharmaceutical companies committed to facilitating rigorous and timely research that gets to the heart of pressing health policy issues.
NPC does not engage in political advocacy. NPC conducts health policy research, which is frequently published in respected peer-reviewed journals. NPC also engages regularly in multistakeholder initiatives, conferences, and other industry dialogues.
Each member company is represented on NPC’s Board of Directors and helps to inform the research agenda through participation on various Board-level committees, the Research Work Group, and the Policy and Communications Work Group or through Corresponding Officers.
In addition to our public resources, NPC develops materials exclusively for members — examples include special issue briefs detailing the impact of developments in the healthcare landscape on the biopharmaceutical industry. We also provide educational resources, practical tools, analytical papers, access to expert consultation, and support for member meetings.

Board of Directors
EXECUTIVE COMMITTEE
Christine G. Marsh (Chair)
Senior Vice President, Market Access
Boehringer Ingelheim Pharmaceuticals, Inc.
Christian Nguyen (Vice Chair & Treasurer)
Senior Vice President, Value, Evidence and Outcomes
Eli Lilly and Company
Jeremy Allen (Member-At-Large)
Head, Government Affairs
Spark Therapeutics, Inc.
Shontelle Dodson (Member-At-Large)
Executive Vice President, Medical Affairs Head
Astellas Pharma, Inc
OTHER MEMBERS OF THE BOARD
Dana Lawlor
Vice President, Market Access Strategy
AbbVie
Ruth Hoffman
Executive Director, US Health Policy Strategy and Industry Trends
Amgen, Inc.
Mohit Manrao
Senior Vice President, Head of US Oncology
AstraZeneca
Edward W. Feeley
Senior Vice President, Head of Market Access
Bayer U.S. LLC
Chris Leibman
Senior Vice President, Head of Value and Access & Public Policy and Government Affairs
Biogen
Chris Mancill
Senior Vice President and Head, Worldwide Value, Access, Payment & Health Economics and Outcomes Research
Bristol Myers Squibb
Jan E. Hansen
Vice President, Evidence for Access Medical Unit
Genentech, Inc.
Rekha Ramesh
Vice President and Head of Global Policy
Gilead Sciences
Beth Rada
Vice President, Government and Public Affairs
Horizon Therapeutics
Blasine Penkowski
Chief Strategic Customer Officer, Janssen North America
Johnson & Johnson
Kate Robinson
Vice President, US Market – Integrated Account Management
Merck
Courtney Piron
Vice President and Head, US Public Affairs and US Country President
Novartis Services, Inc.
Rick Dudek
Senior Vice President, US Market Access
Pfizer Inc.
Trent McLaughlin
Executive Director, Global Market Access & Evidence
Sarepta Therapeutics
Phil Naughten
Vice President, Value & Evidence Generation, US Medical
Takeda Pharmaceuticals U.S.A., Inc.
Patty Fritz
Head, U.S. Corporate Affairs
UCB
John M. O’Brien (ex officio)
President and CEO
National Pharmaceutical Council
THE NPC TEAM
As of December 31, 2023
John M. O’Brien, PharmD, MPH, President & Chief Executive Officer
Jonathan D. (Jon) Campbell, PhD, Chief Science Officer
Kimberly Westrich, MA, Chief Strategy Officer
Michael Pratt, Chief Communications Officer
Robert J. Nordyke, PhD, Vice President of Research
Julie Patterson, PharmD, PhD, Senior Director of Research
Tyler D. Wagner, PharmD, PhD, Associate Director of Research
James Motyka, PharmD, Research Associate
Gabri’el Shabazz, MPH, Research Coordinator
Rayan Salih, PharmD, Rutgers-NPC 2023 - 2025 Health Policy & Communications Fellow
Tanya Bailey, MS, Head of Membership and Meetings
Sue Grimes, Executive Assistant
Nicole Reynolds Berry, Director of Administration
