2024 NPC Annual Report
Leadership Messages
President's Message
 In 2024, NPC proudly celebrated its 70th year of conducting policy-relevant research that informs conversations and decision-making and supports biopharmaceutical innovation that meets the needs of patients.
In 2024, NPC proudly celebrated its 70th year of conducting policy-relevant research that informs conversations and decision-making and supports biopharmaceutical innovation that meets the needs of patients.
In an era of stark contrasts, NPC’s work has never been more important. Every day, NPC members transform the lives of patients by driving scientific advances and translating them into new treatments; and yet public policy conversations continually fail to understand and increasingly undermine the drug development ecosystem. Policies intended to improve affordability have unintentionally threatened access to lifesaving treatments and cures for patients.
Over the last year, NPC navigated an increasingly complex and dynamic health policy environment to publish policy-relevant research and communicate it with impact. A growing and talented team generated research, analysis, and commentary on the most important issues: the unintended consequences of the IRA and government price setting, the impact of the 340B program, the shortcomings of value assessment, and the perverse incentives of PBMs that drive health plan utilization management. Our work was powered by a highly engaged and experienced Board of Directors and member company colleagues driven by the desire for more people living with disease to benefit from medicines that help them get well, stay healthy, and avoid more costly settings of care.
As we head into 2025, NPC will meet the challenges of a new administration, a new Congress, and new health policy debates with a new strategic plan informed by the renewed thinking of an engaged membership. Our work will anticipate NPC members’ needs and nimbly respond to new challenges with timely evidence and insights. And above all else, NPC will work to create a healthcare system where advances in medicine are accessible to patients, valued by society, and sustainably reimbursed by payers to ensure continued innovation.
I invite you to join us on that journey.
John M. O’Brien, PharmD, MPH
President and CEO
Board Chair's Message
 Every biopharmaceutical company carries a unique mission, but the member companies of NPC come together with a shared commitment to driving research that recognizes the importance of biopharmaceutical innovation for patients.
Every biopharmaceutical company carries a unique mission, but the member companies of NPC come together with a shared commitment to driving research that recognizes the importance of biopharmaceutical innovation for patients.
Decision-makers must navigate a complex healthcare delivery system, whether they work in state and federal legislatures, regulatory agencies, large employers or commercial health plans, value assessment organizations, or professional societies. NPC recognizes that their decisions impact not just patient access to treatments that are available today, but also the development of new treatments tomorrow.
For 70 years, NPC’s research has served as an essential resource that equips decision-makers with evidence-based insights that can help them better understand the value of medicines to patients, their communities, and the healthcare system as a whole. Over the course of 2024, industry encountered new and growing challenges in the health policy landscape. I am proud that, over my term as Board Chair, we were able to meet the moment by growing NPC’s research portfolio and addressing emerging questions in a rapidly shifting landscape.
The strength of NPC’s work lies in the close collaboration between the Board and the NPC team, which allows us to share expertise and experiences, identify emerging trends and issues, and drive a research agenda that provides timely insights for all those engaged in critical health policy conversations. NPC’s work recognizes the stakes of day-to-day policy decisions, which will either help or hinder the effort to bring life-enhancing medicines to patients today, tomorrow, and for generations to come. By continuing to equip decision-makers with sound evidence, NPC and its research will remain an invaluable guidepost for driving progress for patients.
Christine Marsh, MBA
Board Chair, NPC
Senior Vice President, Value & Access, Boehringer Ingelheim
Incoming Board Chair's Message
 As we head into 2025, there is a lot that is new and uncertain — not the least of which is new leadership and policy agendas in Washington, D.C., and in statehouses across the country.
As we head into 2025, there is a lot that is new and uncertain — not the least of which is new leadership and policy agendas in Washington, D.C., and in statehouses across the country.
One thing that hasn’t changed: quality, policy-relevant research and effective communication of the data and evidence are critical. This is even more true given the policy conversations ahead of us in 2025.
NPC has an important role in generating that evidence. As I reflect on my time serving on NPC’s Board over the past several years, I am reminded of the increasing importance of NPC's work: NPC generates policy-relevant research and communicates it with impact, educating decision-makers and informing policy conversations.
I believe NPC’s mission, leadership, and plan are all uniquely fit for the moment we are in. It is an honor to step into the role of Board Chair for NPC at such a pivotal time. I look forward to working with all of you to continue to bring together diverse perspectives, foster a more informed dialogue, and generate rigorous research to guide decision-making.
Together, we can help ensure biopharmaceutical breakthroughs continue to arrive and that the healthcare system allows patients to access them.
Jen Norton
Incoming Board Chair, NPC
Senior Vice President, Head of U.S. Patient and Market Access, Takeda
NPC in 2024
Policy Relevant Research Communicated With Impact
In 2024, the implementation of the Inflation Reduction Act’s Medicare Drug Price Negotiation Program (DPNP) continued to dominate the health policy landscape, but it was accompanied by growing discussion around other critical issues. The explosive growth of the 340B program drew more questions about the scale of abuse and the costs to employers, patients, and taxpayers. The rise of state-based prescription drug affordability boards (PDABs) made the discussion about government price controls a local issue. Policymakers continued to scrutinize the practices of pharmacy benefit managers (PBMs) and the impact on costs faced by patients and employers. And continued advances in bringing transformative new treatments to patients with conditions like cancer, liver disease, sickle cell disease, schizophrenia, diabetes, and obesity served as a vivid backdrop for discussions about assessing the value of treatments and the impact of health benefit design and utilization management on patient access.
At NPC, everything starts with evidence and science. In 2024, NPC continued to focus on three strategic priority areas: to examine and explain the true cost of medicines and the value they provide; to address critical issues of value assessment and other approaches used to impact drug pricing and the downstream effect on innovation; and to educate policy stakeholders and the public on the underlying issues that impact patient access. NPC also published important work exploring the impact of the IRA’s Medicare DPNP and other government interventions.
Our research agenda was informed through more interactions with members and external stakeholders to better understand what research questions were most relevant. Our team cut cycle time so the research we published was more timely. And we re-launched the Employer Work Group as we continued to develop research relevant to employers who play an important role in health benefits for millions of Americans.
As a result, NPC’s research informed policy conversations throughout the year. It was cited by patient groups, mentioned during Congressional testimony, circulated on the Hill, covered by the media at important policy moments, “tweeted” by Mark Cuban, and shared by key opinion leaders in their thought leadership. More leaders and decision-makers consumed NPC newsletters and LinkedIn thought leadership than ever before. We also connected directly with patient advocacy leaders and alliance organizations to share our research and to understand their priorities.
The NPC team also shared its research findings with relevant audiences from stages and podiums at industry, professional society, and academic conferences. The robust speaking calendar meant that stakeholders heard our research presented by a chorus of NPC voices — and the research presented from these increasingly larger and more prominent stages received more coverage.
NPC also continued to invest in the next generation of health policy leaders. In July, the NPC team welcomed a second Rutgers-NPC Fellow, Haley McKeefer, PharmD, MHA, MS, who joined senior fellow Rayan Salih, PharmD, on this accelerated path to industry experience. NPC also launched a Summer and Fall Scholars Program for undergraduate and early-career individuals interested in health policy-related research and communication, and sponsored student and early-career receptions at important conferences hosted by organizations such as ISPOR and AMCP.
In addition, members of the NPC team were invited to engage with the student community throughout the year. Dr. O’Brien delivered the hooding ceremony address to the Washington State University’s College of Pharmacy and Pharmaceutical Sciences Class of 2024, guest-lectured with former Health and Human Services Secretaries Donna Shalala and Alex Azar at the University of Miami's Herbert Business School, and was joined by members of the NPC team at lectures and student association presentations at Rutgers University's Ernest Mario School of Pharmacy, the Medical University of South Carolina’s College of Pharmacy, and Nova Southeastern University's College of Pharmacy, among others.

Finally, NPC continued to convene leaders to better understand the risk of public policy and inform a policy-relevant research agenda. NPC hosted several webinars with leaders from patient organizations to discuss research related to topics such as the Accelerated Approval Program, and the NPC Leadership Forum series convened policy and industry leaders for frank conversations on the policy landscape throughout the year for the benefit of our members.
NPC’s work in 2024 was underpinned by a commitment to invest in our people and in our culture.
Building a Team to Meet the Moment
 NPC added to its leadership team by appointing Tanya Bailey, MS, as Chief of Staff. Ms. Bailey first joined NPC in 2015. Bringing her deep knowledge of member companies and their priorities, she is driving the NPC team’s execution against its strategic plan and leading initiatives in membership engagement and educational events and meetings.
NPC added to its leadership team by appointing Tanya Bailey, MS, as Chief of Staff. Ms. Bailey first joined NPC in 2015. Bringing her deep knowledge of member companies and their priorities, she is driving the NPC team’s execution against its strategic plan and leading initiatives in membership engagement and educational events and meetings.
- NPC named Julie Patterson, PharmD, PhD, as Director of the NPC Enterprise Lab, overseeing NPC’s in-house research projects. The establishment of the Enterprise Lab increased the relevance and decreased the timeline to publication of NPC’s research in 2024.
- Jacquelyn (Jackie) McRae, PharmD, MS, joined NPC as Vice President of Policy and External Affairs, leading work to bridge NPC’s communications, policy, and research portfolios through strategic communications and health policy activities.
- Katherine Lovro joined NPC as Senior Director of Alliance Development to expand NPC’s strategic partnerships and educational collaborations with patient advocacy groups, professional associations, and other stakeholders.
- Devon Bortz joined NPC as Strategic Communications and Marketing Manager, supporting NPC’s work to communicate research findings and engage with health policy audiences through thought leadership, educational resources, and NPC’s owned and earned channels.
- Hanke Zheng, MS, PhD, joined NPC as a Research Manager to increase the capabilities of our internal research team.
- James Motyka, PharmD, was promoted from Research Associate to Research Manager, as part of growing NPC’s internal research capabilities.
Investing in Our Culture
An experienced and skilled team is critical to NPC’s success and a strong workplace culture is essential to bringing out the best in each individual. Through culture initiatives led by Chief Strategy Officer Kimberly Westrich, NPC’s leadership team has prioritized and invested in an environment that fosters each team member’s strengths and enables individuals to fully contribute to the organization’s success.

This year, NPC focused on three key areas:
- Strengthening the Foundation: By supporting professional development workshops and interpersonal growth opportunities, NPC nurtured the foundation necessary for a supportive workplace environment. The workshops inspired collaborative conversations, deepened mutual trust, and fostered creativity.
- Establishing a Common Language: NPC offered opportunities for the team to explore and optimize communication across teams. Team members engaged in workshops and conversations to establish frameworks for delegation and delivering feedback, as well as strengthening interpersonal interactions informed by insights from DiSC®.
- Continuous Learning: By committing to an ongoing dialogue and creating feedback loops, NPC has built a continuous growth and learning model that views workplace culture as a work-in-progress rather than a finished product.

 |
NPC’s commitment to supporting growth and excellence was recognized in 2024 when NPC was certified as a Great Place To Work®. This achievement reflects NPC’s ongoing commitment to investing in building and maintaining a healthy workplace culture. |
Research Highlights
NPC published policy-relevant research in 2024 across NPC’s three strategic priority areas and presented research at conferences throughout the year in all of these areas.
The number and relevance of the publications from the research team allowed NPC to inform health policy conversations, addressing new and emerging questions in a rapidly shifting landscape. Members of the research team, led by Dr. Campbell, conducted research briefings with member companies, policy organizations like the Congressional Budget Office, patient organizations, and academic institutions.
January – March
 |
Association between adherence with oral anticancer medications and short-term health care resource utilization: A 2010-2018 claims-based analysis – Published in the Journal of Managed Care and Specialty Pharmacy (JMCP), this study examined the effect of adherence to oral anticancer medications on health care resource utilization (HRU) among patients with cancer. |
 |
Guiding Practices for Patient-Centered Value Assessment (2024) – NPC published an update of its guiding practices for U.S. value assessment to help inform the growing discussion of this tool and to support pricing pharmaceuticals based on the value they provide to patients and society as a whole. |
 |
Unintended Consequences of the Inflation Reduction Act: Clinical Development Toward Subsequent Indications – In a study published in the American Journal of Managed Care (AJMC), NPC’s research team explored how the IRA might impact the clinical development for subsequent indications for high-spend Medicare Part D small molecule drugs. The research demonstrated how the IRA may reduce economic incentives to develop multiple indications, including single-indication launches and investments in postapproval research for additional indications. |
 |
Medicare Part D Coverage of Drugs Selected for the Drug Price Negotiation Program – Published in JAMA Health Forum, this study found that current Medicare beneficiary access to the drugs selected for the first round of the IRA’s Medicare DPNP was high in 2019 and 2023, and that perverse incentives created by the IRA encourage increased utilization management and could reduce patient access to treatments. |
 |
The Cost of the 340B Program Part 1 & Part 2 – NPC supported these reports published by IQVIA examining the hidden costs of the 340B program to employers. Health costs increased for self-insured employers and their employees by $5.2 billion annually thanks to the 340B program. Plus, the study found that revenue-sharing arrangements with 340B hospitals drive up costs for everyone except the hospital. |
 |
Specialty drug use for autoimmune conditions varies by race and wage among employees with employer-sponsored health insurance – Published in JMCP, this NPC study found that low-income and non-white individuals enrolled in employer-sponsored health insurance have lower usage of specialty drugs for autoimmune conditions. The findings suggest that employers seeking to manage specialty medicine costs through increased cost-sharing may contribute to disparities in use. |
April – June
 |
The Impact of Patient Copay Accumulators and Maximizers on Out-of-Pocket Costs and Medication Persistence – Featured at #AMCP2024, this NPC research explored the impact of copay accumulators and maximizers on patients. The research demonstrated that these mechanisms increase OOP costs for patients and lead more patients to abandon prescribed medications, compared to patients with standard benefit designs. |
Presented at #ISPOR2024
|
Variation in per Beneficiary Prescription Utilization and Spending By Race/Ethnicity in Medicare and Medicaid Insurance Claims – This study delved into the question of how prescription drug spending and use is distributed across the U.S. population. The research team found that substantial variation exists in Medicare and Medicaid prescription utilization and spending by race/ethnicity. |
| Mapping Medicaid: A Comparative Analysis of State-Level Racial and Ethnic Data Collection to Federal Guidelines – This research evaluated the consistency of state-level race and ethnicity applicant questions and assessed adherence to these federal directives to reinforce data integrity in healthcare policymaking. Authors found a striking variance in race categories and divergence between OMB and HHS guidelines and noted the importance of improving race and ethnicity data to crafting health policies that truly encompass America's diversity. | |
| Beyond the Headline: Inflation-Adjusting ICER’s Unsupported Price Increase Results – This study compared ICER’s unadjusted findings to inflation-adjusted findings and found that by not accounting for inflation, ICER overestimates the impact of price increases on drug spending. The findings call into question the value of ICER's UPI reports for informed policy-making. | |
| Employer Perspectives on Alternative Funding Programs – This survey-based study explored the controversial practice of alternative funding programs (AFPs) tapping into patient advocacy groups, foundations, or grants to pay for patients’ medication costs. Most survey respondents took a neutral stance on AFPs but recognized that aspects like diverting resources from under-insured patients can be problematic. | |
 |
Breadth of Patient and Stakeholder Input in CMS's Drug Price Negotiation Program: A Content Analysis of the 2023 Patient-Focused Listening Sessions – This NPC research was published in Value in Health and described the breadth of patient and stakeholder input during CMS-hosted sessions. The analysis found that CMS heard less than 15 minutes of patient-focused input per session and that they fell short of capturing voices of diverse and Medicare-specific patient populations. |
 |
Unanswered Questions And Unintended Consequences Of State Prescription Drug Affordability Boards – NPC published a primer in Health Affairs Forefront focusing on the uncertainties, challenges, and unintended consequences for patients amid the varying approaches to implementing upper payment limits through PDABs. |
July - September
 |
Prescription Rebate Guarantees: Employer Insights – This research published in AJMC captured employers’ perspectives of rebate guarantees, dependency upon rebate dollars, and the role that pharmaceutical rebates or employer benefits consultants play in their pharmacy benefits manager (PBM) selection. |
 |
The Accelerated Approval Program for Oncology Drugs: Celebrating More Than 250,000 Life-Years Gained and Counting – NPC’s commentary in AJMC examined evidence regarding the clinical benefit of the FDA’s Accelerated Approval Program and found that the program is working as intended and for the benefit of patients. |
October - December
 |
The Inflation Reduction Act and Drug Development: Early Signals of Impact on Post-Approval Clinical Trials – At the inaugural Washington Health Economics Symposium, NPC’s Jon Campbell presented research on the potential unintended consequences of the IRA on drug development, outlining how subsequent indications and additional rare disease clinical development are disincentivized under the IRA and identifying potential policy proposals in discussion. |
 |
Will the Institute for Clinical and Economic Review’s Shared Savings Approach Decrease Value-Based Prices Most for the Most Severe Diseases? – This study published in Value in Health confirmed that the use of “shared saving” methods will diminish innovation incentives in areas of high unmet need. |
 |
Subsequent Indications in Oncology Drugs: Pathways, Timelines, and the Inflation Reduction Act – Published in Therapeutic Innovation & Regulatory Science, this study explores how changes to the incentives for drug development due to the IRA’s DPNP could impact post-approval development in cancer drugs. Authors found that a quarter of all drugs assessed in the study were approved for their most recent subsequent indication after the time they would be DPNP-eligible. Drugs with the most rapid pace of development between indications are at the most risk of delayed initial launches, while those with more measured development timelines may be most at risk of delaying or suspending research on subsequent indications. |
 |
Alignment of Commercial Health Plan Specialty Drug Utilization Management Criteria With Clinical Treatment Guidelines – Presented at AMCP Nexus, this study investigated the relationship between commercial health plans’ utilization management criteria and published clinical guidelines. Authors found a misalignment between clinical guidelines and health plan UM in specialty drug coverage policies and that plans often cover drugs more narrowly than the FDA’s approved indication. |
 |
CMS’s Drug Price Negotiation Program 2023 Patient-Focused Listening Sessions: A Descriptive Analysis of Speaker Remarks – NPC’s research team analyzed discussions during the series of patient-focused listening sessions held for each of the first 10 drugs selected for the Inflation Reduction Act’s Medicare Drug Price Negotiation Program (DPNP). The study, published in Pharmacoeconomics - Open, details learnings to help CMS improve the format and speaker representativeness of future patient-focused listening sessions. |
 |
Improving Access to Gene Therapies: A Holistic View of Current Challenges and Future Policy Solutions in the United States – Published in the Journal of Comparative Effectiveness Research (JCER), this study provides a more holistic multistakeholder examination of the unique policy challenges associated with innovative gene therapies and explores opportunities for addressing those challenges. |
 |
Inflation-Adjusted Analysis of ICER's Unsupported Price Increase Reports: Contextualizing Drug Spending Changes – Published in the Journal of Medical Economics (JME), this study evaluated the economic context of “unsupported” drug price increases reported by ICER and found that the lack of inflation adjustments in its assessments resulted in misleading estimates of real changes in healthcare spending across all drugs analyzed. When ICER released it's 2024 UPI Report, NPC quickly published a response inflation-adjusting the 2024 headline number, saying "It is time for ICER to retire this fundamentally flawed report that misleads stakeholders and risks driving harmful policy decisions." |
Sharing Our Research at Convenings

Led by Dr. O’Brien and Dr. Campbell, the NPC team brought research findings and analysis to numerous industry events, academic institutions, and professional society conferences in 2024. At these convenings, NPC offered timely insights on the IRA and its unintended consequences, expert analysis of CMS’s approach to implementation and the role of patient input, reviews of PDABs emerging in states around the country, perspectives on PBM reform, recommendations for improved 340B transparency and potential outcomes, and discussions on pressing issues related to health technology assessment, value-based care, formulary design, patient access, health equity, and more. By engaging directly with critical audiences, NPC was able to foster evidence-guided conversations throughout the year.

January - March Conference Spotlights
2024 ACCESS Forum International Annual Meeting & Expo
At the 2024 ACCESS Forum International Annual Meeting and Expo in Miami, Dr. O’Brien participated in a panel session on “The IRA and Its Impact in the U.S. and Beyond.” He was joined by Lisa Nelson of Novartis, former NPC Scholar in Residence Sean Sullivan, Jason Spangler of the Center, and Nancy McGee of Lumanity. The conversation explored how the law’s Medicare DPNP will shift the dynamics for payers offering Medicare Part D plans and how those changing dynamics will impact access for patients. NPC Board Member Chris Mancill of Bristol Myers Squibb and NPC member Johnson & Johnson’s Bridget Doherty also participated in important panels at the conference.
5th Annual IVI Methods Summit
Dr. Campbell joined a panel at the 5th Annual Methods Summit of the Center (formerly IVI, the Innovation and Value Initiative) to discuss “Is Real Option Value Really an Option for Today’s Health Technology Assessment?” The event brought solution-oriented idea exchanges among not only methodologists, but also patients, patient advocates, manufacturers, and others. Dr. Campbell was pleased to connect with NPC members, research partners, and passionate patients and patient advocates.
April - June Conference Spotlights
AMCP 2024 Annual Meeting
At the Academy of Managed Care Pharmacy’s 2024 Annual Meeting, the NPC research team shared their takeaways with media outlets. In a video from The American Journal of Managed Care, Ms. Westrich shared how much she loved getting to interact with the next generation of researchers at #AMCP2024. Meanwhile, Dr. Motyka told Managed Healthcare Executive about the research he presented on the impact of copay accumulators and maximizers on patient out-of-pocket costs and medication persistence. View the poster here.

Asembia's Specialty Pharmacy Summit
Dr. O’Brien addressed the future of value-based contracting for payers at #ASX24 alongside Emily Colaizy of Prime Therapeutics, Ross Hoffman formerly of Centene Corporation, Kent Rogers of ARCH Venture Partners, Lucy Ruwitch Langer of UnitedHealth Group, Jayson Slotnik of Health Policy Strategies, Inc., and Burt Zweigenhaft of FFF Enterprises and the Association for Value-Based Cancer Care.
ISPOR 2024
The NPC team had a strong presence at #ISPORAnnual, presenting multiple posters, speaking at spotlight panel presentations, hosting networking opportunities for future leaders, and sharing research insights with journalists. The team addressed a range of topics with NPC’s research including ICER’s Unsupported Price Increase report, alternative funding programs, patient input on CMS’s Drug Price Negotiation Program, the costs of the 340B program to employers, and others.

Financial Times Live U.S. Pharma and Biotech Summit
Dr. O’Brien participated in the U.S. Pharma and Biotech Summit hosted by Financial Times Live and Endpoints News, joining a panel conversation with Michael Peel of Financial Times Live and Sarah Emond of ICER. The group discussed the current misaligned incentives in the healthcare marketplace and connected with several potential members throughout the course of the day.

2024 BIO International Convention
During a panel session at #BIO2024, Dr. O’Brien shared takeaways from NPC’s primer on PDABs and findings on the impact of federal legislation on state-level prescription pricing practices. Dr. Campbell joined a panel on reimbursement for digital therapeutics, highlighting the need for innovative payment mechanisms to keep up with innovative therapies.


July - September Conference Spotlights
2024 Fierce Biotech Summit
In Boston, Dr. O’Brien shared the Fierce Biotech Summit stage with Christie Bloomquist of Astellas, Andrew Kaplan of Takeda, and Joy Russell of Genentech to discuss the health policy environment heading into 2025.

October - December Conference Spotlights
Washington Health Economics Symposium
Presenting to an enamored crowd at the inaugural Washington Health Economics Symposium, Dr. Campbell unveiled NPC’s research on the potential unintended consequences of the IRA on drug development. Dr. Campbell also joined a panel regarding “Incentives for Research and Development.”
AMCP Nexus 2024
NPC shared policy-relevant research at AMCP Nexus in Las Vegas:
- Ms. Westrich moderated an expert panel discussion on employer utilization of alternative funding programs with media coverage from Managed Healthcare Executive and AJMC. She also spoke at the Corporate Member Council Meeting, informing conversations about the potential policy impact of the upcoming election.
- VP of Policy and External Affairs Dr. McRae gave opening remarks at our sponsored Student and New Practitioner Networking Reception. We connected with over 50 emerging professionals and seasoned industry leaders, affirming our dedication to the next generation of health policy leaders.
- Dr. Motyka presented a poster, in collaboration with researchers from the Tufts Center for the Evaluation of Value and Risk in Health, on the alignment of commercial health plan utilization management criteria with clinical treatment guidelines. View the poster here.
Annual Association for Value-Based Cancer Care (AVBCC) Summit and Educational Program
During three sessions at the 14th Annual Association for Value-Based Cancer Care Summit and Educational Program in New York City, Dr. O’Brien shared NPC research findings on the IRA’s impact on subsequent indications for oncology drugs and his thoughts on the impact of policy on the oncology ecosystem, the potential implications of the 2024 election, and the outlook for 2025.
Galien Forum and Prix Galien Awards 
At the Galien Forum USA 2024, Dr. O’Brien shared NPC's research findings in a panel discussion on PBMs, pricing, access, and the global supply chain. The panel was part of spirited conference discussions with Nobel laureates, top policy makers, and leading industry executives who gave perspectives on the most significant health challenges of our time. The NPC team celebrated the many member companies nominated for distinguished awards recognizing their important contributions to improving treatment options for patients. Congratulations to NPC members Pfizer, who won “Best Pharmaceutical Product” for Paxlovid, and AstraZeneca, who won “Best Biotechnology Product” with Daiichi Sankyo US for Enhertu.
ACCESS Forum EU ‘24 
In Barcelona, Dr. Campbell presented insights on the implications of the IRA’s Medicare Drug Price Negotiation Program, engaging market access leaders worldwide with NPC’s IRA research. NPC then co-hosted a reception with ACCESS Forum and ISPOR to connect with colleagues and develop fresh ideas for generating good evidence for good decision-making.
In his panel, Dr. O’Brien remarked on how biopharma is adapting to challenges in the life sciences industry alongside Eric Faulkner of SymetryML, Passage Health Associates, and the NAMCP Medical Institute, Jonathan Kowalski of Lumanity, Vivian Mendonca of the Menarini Group, and Dustin O’Dell of SymetryML.
ISPOR Europe 2024
As ACCESS wrapped and ISPOR Europe began, NPC co-hosted a joint reception with ACCESS Forum and ISPOR gathering some of the top HEOR leaders for an evening of connecting, camaraderie, and conversations.
Dr. Campbell joined a member event during the ISPOR Europe 2024 conference to provide insights on the role of health economics and outcomes research and value assessment going forward and to share NPC’s research and perspective on value assessments as a tool, not a rule.

Alliance for Health Policy’s 2024 Post-Election Symposium
Dr. O’Brien shared insights on which issues would be top of mind for the incoming administration in a post-election panel with Marc Samuels of ADVI, Adam Colborn of AMCP, and Rachel Nuzum of The Commonwealth Fund.

National Alliance Annual Forum
Both Dr. O’Brien and Ms. Westrich amplified crucial NPC research findings at the National Alliance Annual Forum, which took place in November in Arlington, VA.
- Ms. Westrich led an important workshop on the PBM Misalignment Initiative alongside Leta Evaskus of the Washington State Health Care Authority, Carrie Rust of ELLWOOD Group Inc., Jeffrey Townsend of HealthcareTN, and Renzo Luzzati of US-Rx Care. During the session, Ms. Westrich shared NPC’s exploration of employer perspectives of rebate guarantees, dependency upon rebate dollars, and the role that pharmaceutical rebates or employer benefits consultants play in their PBM selection.
- Dr. O’Brien participated in a panel session on “Unlocking Value: Buildings Trusted Tools to Assess High-Value Care.” Sarah Emond of ICER, Caroline Pearson of the Peterson Center on Healthcare, Shawn Gremminger of the National Alliance of Healthcare Purchaser Coalitions, Nilay Shah of Delta Air Lines, and Dr. O’Brien discussed essential tools and strategies to evaluate cost-effectiveness, clinical outcomes, and overall value.

External Affairs
With the growth of the external communications team in 2024, NPC expanded its capacity to bring research to key audiences — leveraging research to deliver crucial policy analysis, foster industry dialogue, and drive stakeholder engagement on complex policy issues. The results of the team’s efforts were visible in the larger dialogue around the IRA, drug pricing, PBMs, 340B, and other pressing topics, as industry thought leaders, policymakers, and journalists increasingly sought out and cited NPC research.
In February, a member of Congress cited NPC’s research on the unintended consequences of the IRA's Medicare DPNP in their statement on Rare Disease Day.
NPC’s research on 340B was twice cited in Congressional testimony as part of a June 4 hearing held by the House Subcommittee on Oversight and Investigations of the Committee on Energy and Commerce on “Oversight of the 340B Drug Pricing Program.”
NPC’s research is frequently cited by patient and alliance groups and by key opinion leaders. It also informs policy conversations in the popular press.
Throughout the year, health policy reporters drew on insights from NPC’s research and experts during critical policy milestones — particularly during important stages of CMS’s implementation of the Medicare DPNP. The coverage was a result of thoughtful engagement with journalists working to provide in-depth coverage and analysis in a new and shifting landscape.

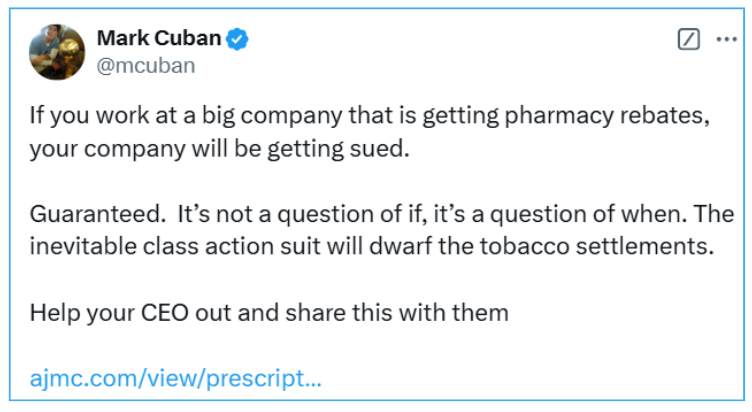 Our research also reached influential business leaders this year: for example, Mark Cuban shared our research on rebate guarantees and the role of employer benefits consultants in PBM selection with business leaders. He recommended our work on X to CEOs the same week as the July PBM Hearing on Capitol Hill.
Our research also reached influential business leaders this year: for example, Mark Cuban shared our research on rebate guarantees and the role of employer benefits consultants in PBM selection with business leaders. He recommended our work on X to CEOs the same week as the July PBM Hearing on Capitol Hill.
NPC continues to serve as a key resource for advocacy organizations working to assess how new policies and shifting dynamics impact the patients they serve. Throughout the year, the team frequently met with organizations to share research findings and acquire insights that could help inform the questions being explored in NPC’s research pipeline.
As both the volume of NPC’s research and the appetite for timely insights have grown, so has NPC’s community of followers and subscribers. NPC has expanded its efforts to bring timely news and information to audiences who need access to relevant news in an easily digestible format — resulting in increased readership of “NPC This Week,” Kimberly Westrich’s weekly “Value Viewpoint,” and the regular highlights shared by NPC on LinkedIn.
In 2024, NPC advanced an important member benefit — engagement with leading voices in health policy through Leadership Forums hosted throughout the year. These educational programming opportunities offer NPC’s Board of Directors, Corresponding Officers, and Work Group members a platform to share knowledge, collaborate, and help foster innovation and best practices.
This year, NPC Leadership Forums convened experts on several pressing topics, including:
- The challenges employers face when purchasing healthcare benefits, and a discussion of potential solutions, new offerings, and the legal landscape on the table.
- The impact of the IRA on the pharmaceutical supply chain in Medicare Part D and Part B.
- Learnings from research about drug pricing policies and what to expect from the Medicare Drug Price Negotiation Program.
- The current health policy environment in an election year and beyond.
- A discussion on value and access, specifically whether value assessment has reached an inflection point in the U.S., and the role of value and health technology assessment in U.S. health policy and patient access.
- A post-election health policy outlook for 2025 the week after the November election.
You can also subscribe to see NPC This Week in your LinkedIn feed. Subscribe on LinkedIn
Membership and Board
NPC is a member-based nonprofit organization supported by leading biopharmaceutical companies committed to facilitating rigorous and timely research that gets to the heart of pressing health policy issues.
NPC does not engage in political advocacy. NPC conducts health policy research, which is frequently published in respected peer-reviewed journals. NPC also engages regularly in multistakeholder initiatives, conferences, and other industry dialogues.
Each member company is represented on NPC’s Board of Directors and helps to inform the research agenda through participation on various Board-level committees, the Research Work Group, Employer Work Group, and the Policy and Communications Work Group or as Corresponding Officers.
In addition to creating and distributing public resources, NPC develops materials exclusively for members — such as special issue briefs detailing the impact of developments in the healthcare landscape on the biopharmaceutical industry. NPC also provides educational resources, practical tools, analytical papers, access to expert consultation, and support for member meetings.

2024 Board of Directors
Chair
Christine G. Marsh
Senior Vice President, Value and Access
Boehringer Ingelheim Pharmaceuticals, Inc
(from July 2024)
Christian Nguyen
Senior Vice President, Value, Evidence and Outcomes
Eli Lilly and Company
(prior to July 2024)
Vice Chair
Jen Norton
Senior Vice President of US Patient and Market Access
Takeda Pharmaceuticals U.S.A., Inc.
(from July 2024)
Jan E. Hansen
Vice President, Evidence for Access
Genentech, Inc.
(prior to July 2024)
Treasurer
Rekha Ramesh
Vice President and Head of Global Policy
Gilead Sciences
Members at Large
Jeremy Allen
Head, Government Affairs
Spark Therapeutics, Inc.
William “Bruce” Wilson
Vice President, U.S. Oncology Care & Access
AstraZeneca
OTHER MEMBERS OF THE BOARD
(as of December 1)
Ruth Hoffman
Executive Director, US Health Policy Strategy and
Industry Trends
Amgen, Inc.
Ryan Munafo
Vice President, Market Access, Head of Account Management
Bayer U.S. LLC
Chris Leibman
Senior Vice President, Head of Value and Access & Public Policy and Government Affairs
Biogen
Annette Powers (Acting Board Member)
Senior Vice President, Global Market Access
Bristol Myers Squibb
Kevin Hern
Senior Vice President of Market Access Strategy and Capabilities for Lilly USA
Eli Lilly and Company
EMD Serono – To Be Announced
Valerie Reynolds
Executive Director, US Policy & Evidence
Genentech, Inc.
Sulabha Ramachandran
Vice President and Head of Real-World Evidence & Health Outcomes Research
GSK
Blasine Penkowski
Chief Strategic Customer Officer, Janssen North America
Johnson & Johnson
Kate Robinson
Vice President, US Market – Integrated Account Management
Merck
Courtney Piron
Vice President and Head, US Public Affairs and
US Country President
Novartis Services, Inc.
Rick Dudek
Senior Vice President, US Market Access
Pfizer, Inc.
Trent McLaughlin
Executive Director, Global Market Access & Evidence
Sarepta Therapeutics
Patty Fritz
Head, U.S. Corporate Affairs
UCB
John M. O’Brien (ex officio)
President and CEO
National Pharmaceutical Council

Learn More About NPC's 2025 Board Leadership
The NPC Team
As of December 1, 2024
John M. O'Brien, PharmD, MPH, President and Chief Executive Officer
Tanya Bailey, MS, Chief of Staff
Jon Campbell, PhD, Chief Science Officer
Kimberly Westrich, MA, Chief Strategy Officer
Michael Pratt, Chief Communications Officer
Nicole Reynolds Berry, Director of Administration
Devon Bortz, Manager, Strategic Communications and Marketing
Sue Grimes, Executive Assistant
Katherine Lovro, Senior Director, Alliance Development
Haley McKeefer, PharmD, MHA, MS, Rutgers-NPC 2024 - 2026 Health Policy & Communications Fellow
Jacquelyn McRae, PharmD, MS, Vice President of Policy and External Affairs
James Motyka, PharmD, Research Manager
Julie Patterson, PharmD, PhD, Senior Director of Research
Rayan Salih, PharmD, Rutgers-NPC 2023 - 2025 Health Policy & Communications Fellow
Tyler D. Wagner, PharmD, PhD, Associate Director of Research
Hanke Zheng, MS, PhD, Research Manager

Meet the NPC Team
Read about the background and experience the NPC team brings to the organization's work.



