As the health care system in the United States continues to transition from volume to value, recent research shows that value-based agreements (VBAs) between payers and biopharmaceutical manufacturers may be more prevalent than previously assumed. Researchers from the Duke-Margolis Center for Health Policy and the National Pharmaceutical Council (NPC) surveyed payers and manufacturers to get a better understanding of the prevalence of these agreements, which are also known as outcomes-based contracts, performance-based contracts, or risk-sharing agreements. The findings also identify the challenges associated with negotiating VBAs as well as some of the success factors for arriving at a successful agreement.
In a two-phase study, which included a survey and follow-up interviews, the researchers gathered information from 9 payers and 11 drug manufacturers. While a small sample, the participants reported significant value-based contracting activity, totaling more than 100 value-based agreements (VBAs) implemented between 2014 and 2017.
In the current environment of driving greater value in the U.S. health system, stakeholders are more interested in developing new partnerships. “If we focus solely on the publicly known VBAs, we greatly underestimate the prevalence and potential impact of these arrangements in the market, and the progress being made to transition from volume to value more generally,” said Robert Dubois, MD, PhD, chief science officer, National Pharmaceutical Council. Recent developments, such as new FDA guidance around the exchange of health care economic information, and the Request for Information issued by the U.S. Department of Health and Human Services on the Anti-Kickback statute, indicate a strong interest in removing long-standing barriers to value-based contracting.

“Our study illustrates the significant interest and activity in value-based contracting,” said Gregory Daniel, PhD, MPH, Deputy Director, Policy for Duke-Margolis Center for Health Policy. “To support the continued advancement and implementation of innovative contracting arrangements that tie actual payments to measures of value, it will be important to address the legal, regulatory and operational barriers to implementing these contracts to make them worthwhile.”
The study also explored the contract development process to better understand where negotiations between payers and manufacturers break down. Respondents indicated that there are significant hurdles to overcome in the negotiation process.
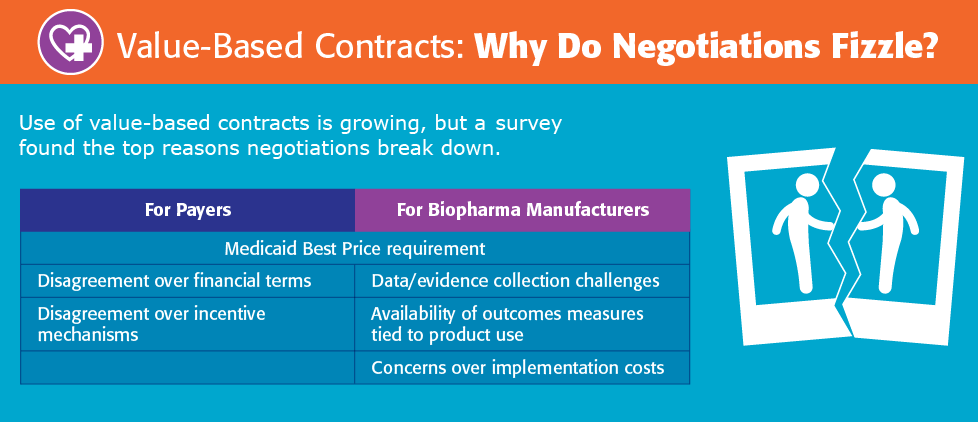
Some of the reasons for negotiation breakdowns include challenges related to data collection and evidence development; the availability of appropriate outcome measures; implementation costs; disagreement over incentive mechanisms; and financial terms. The characteristics that lead to successful negotiations include a target patient population that is easily identified in claims, a reasonable administrative burden, and the availability of measurable outcomes clearly tied to product use.
The study authors suggest that, given the effort required to implement a VBA, future arrangements may benefit from a framework or other evaluative tool that can help manufacturers and payers assess the desirability of pursuing a VBA for a given product and guide negotiations and implementation.
