AUTHORS
Hanke Zheng, PhD, Julie A. Patterson, PharmD, PhD, and Jonathan D. Campbell, PhD of the National Pharmaceutical Council
CONFERENCE
Washington Health Economics Symposium
October 7-8, 2024 (study presented by Dr. Campbell)
ABSTRACT
- Background: Concerns have emerged about the potential unintended consequences of the Inflation Reduction Act (IRA)’s Drug Price Negotiation Program (DPNP) on incentives for drug development, including research towards subsequent indications and post-approval outcomes research. We aimed to understand the impact of the passage of the IRA on the initiation of new Phase I-III clinical trials in previously approved drugs.
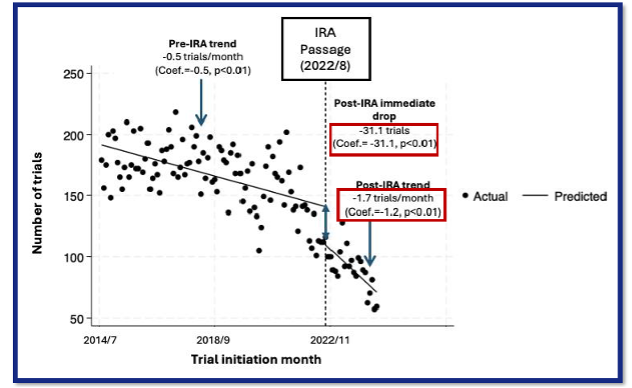
- Methods: This longitudinal study explored the initiation of industry-sponsored clinical trials on previously approved drugs before and after the IRA was passed and signed into law in August 2022. Using the Citeline Trialtrove database, we identified industry-sponsored, post-approval Phase I-III clinical trials that started between July 2014 and June 2024 (i.e., Q3 2014 – Q2 2024). Trials for vaccines were excluded. We summarized the monthly average number of initiated trials during the study period and compared the pre- and post-IRA passage levels using the using the Wilcoxon rank sum test. We then conducted an interrupted time series analysis (ITSA) to evaluate the longitudinal trend in post-approval clinical trial initiation over the study period. Using the ‘itsa’ module in Stata (v18), which performs ITSA via an ordinary least squares regression-based approach, we estimated the impact of IRA passage on the number of and trend in initiated clinical trials.
- Results: A total of 18,208 trials were included in the analysis. The number of clinical trials initiated per month decreased by 45.9% over the study period, with a monthly average [standard deviation, (SD)] of 166.4 (26.1) pre-IRA (July 2014-July 2022) and 90.1 (17.2) after the passage of IRA (August 2022-June 2024) (p<0.01). While 125.6 trials on average per month were initiated in the year preceding IRA passage (August 2021-July 2022), only a mean of 54.8 trials were started per month in the most recently included year (July 2023-June 2024), suggesting a decline of 35.8% within the post-IRA period (p<0.01). The ITSA model indicated a pre-IRA downward trend in post-approval clinical trials, a reduction of 0.5 trials per month (p<0.01) (Figure 1). The IRA further exacerbated the decline by introducing an immediate and significant reduction in trial initiation, by 31.1 trials in one month (p<0.01). Furthermore, the rate of the decline in post-approval clinical trials accelerated following the passage of the IRA, by an additional reduction of 1.2 (1.7 in total) trials per month (p<0.01).
- Research Conclusions: This study provides early evidence of the IRA’s potential impact on investments in post-approval clinical development. Our analysis reveals an immediate and substantial reduction in the number and trend of post-approval clinical trial initiation post-IRA that has persisted through the most recent data available. While the healthcare system continues to recover from the disruptive effects of the COVID-19 pandemic on clinical trial initiation, this analysis that includes COVID-19 in the pre-period provides evidence that manufacturers may already be responding to reduced incentives for investments in post-approval clinical research following the passage of the IRA. Our findings add to the growing body of literature surrounding potential unintended consequences of the IRA on patient access to new treatment options.
- Policy Discussion: This research supports concerns around IRA-related changes to incentives surrounding clinical development. Specifically, subsequent indications and additional rare disease clinical development are disincentivized under the IRA. Policy proposals aligned with these concerns are being discussed, including: extending the IRA’s timeline of small molecule DPNP eligibility to align with that of biologics; delaying eligibility for DPNP selection or removing IRA-imposed inflation rebates when new indications are approved; excluding from DPNP eligibility orphan drugs that treat “one or more rare diseases or conditions”; and developing a transparent, replicable framework that measures and appreciates the comprehensive value of medicines.
Figure 1. Longitudinal Trend of Post-Approval Industry-Sponsored Clinical Trails Pre- and Post-IRA
IRA: Inflation Reduction Act



